An Unknown Rectangular Substance Measures
Lab ane - Density Determinations and Various Methods to Measure Volume
Goal and Overview
This lab provides an introduction to the concept and applications of density measurements. The densities of contumely and aluminum will exist calculated from mass and volume measurements. To illustrate the effects of precision on data, volumes will be adamant by three different methods: geometrically (measuring lengths); water deportation; and pycnometry. The composition of a mixed brass-aluminum cylinder and the book of empty space within a hollow cylinder will also be plant.
Objectives and Science Skills
-
•
Use iii methods to determine the volumes of solid aluminum and solid brass cylinders and assess the relative merits and limitations of each method. -
•
Use mass-based pycnometry measurements to find the volume of a void in a hollow cylinder and the mass fractions of aluminum and brass in a plugged (mixed) metallic cylinder. -
•
Calculate results-based (values and uncertainties) experimental information, known mathematical relationships (e.g., between mass, book, and density), and statistical methods (east.thousand., mistake assay and propagation). -
•
Evaluate the experimental methods and their outcomes in terms of parameters such as reliability, difficulty, accuracy, and precision.
Suggested Review and External Reading
-
•
Data analysis introduction, textbook information on density
Introduction
The density, ρ , of an object is defined as the ratio of its mass to its volume. Density tin be useful in identifying substances. It is besides a convenient property because it provides a link (or conversion gene) between the mass and the volume of a substance.
Mass and volume are extensive (or extrinsic) backdrop of matter - they depend on amount. Density, an intensive (or intrinsic) property, is a kind of "heaviness" cistron. In macroscopic terms, density reflects how much mass is packed into a given three-dimensional space. Typically, densities are reported g/ml or g/cmiii (which are equivalent because 1ml ≡ 1cmiii). Experimentally, mass and volume measurements are required to summate density. Masses are measured on electronic balances. Pan balances, which are accurate to ±0.01 g, are used for quick measurements where greater precision is not required. Analytical balances (accurate to ±0.0001 g) are used for more precise measurements. Volume is an amount of infinite, in iii dimensions, that a sample of matter occupies. The number and the phase of the molecules in the sample primarily determine the book of a substance. Volume will be measured in many ways in this course, only the units are usually milliliters (mL) or cubic centimeters (cmthree). Methods for determining or delivering precise volumes include volumetric pipets and pycnometers; less precise methods include burets, graduated cylinders, and graduated pipets. In this experiment, you lot will measure out masses and volumes to determine density. 4 different metallic cylinders are investigated. In parts 1-3, three different methods are used to observe volume of 2 solid metal cylinders (Al and brass). Each method has its own degree of precision.
(2)
volume by water displacement
(iii)
volume by pycnometry (mass-based)
In parts 4-5, one method for book determination is used to find:
(i)
the volume of a void inside a hollow cylinder; and,
(ii)
the percent composition of a mixed-metal cylinder.
Book by geometry
A cylinder is a standard geometric form. In this case, you tin can measure the dimensions of the cylinder and use the formula to calculate its volume.
( 2a )
V = π
l = d 2 l where d = diameter and 50 = length.
two
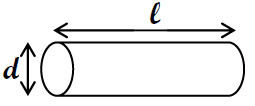
Figure ane
Density would be calculated in 1 footstep to minimize rounding errors:
The doubtfulness in the volume must exist determined by mistake propagation. Mass, length, and bore measurements contribute to the overall uncertainty.
Volume by h2o displacement
For less defined shapes, volume tin can be determined past water displacement. Volumes of liquids such as h2o can be readily measured in a graduated cylinder. To use the water displacement method, an object (in this instance, a small metal cylinder) is inserted into a graduated cylinder partially filled with water. The object's volume occupies space, displacing liquid and raising the water level. The deviation between the ii volumes, earlier and after the object was inserted, is the object'due south volume.
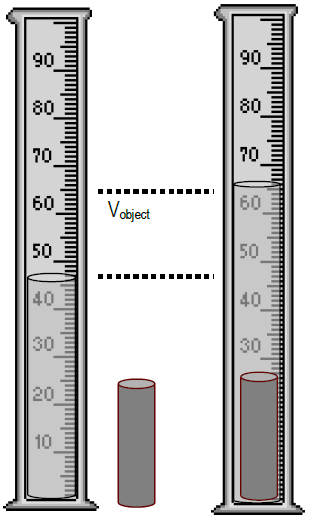
Figure two
( 3a )
V cyl = V terminal − 5 initial = V water + cyl − V h2o
The uncertainty of the volume is based on the 2 volume readings.
( 3b )
σ V cyl = σ V final + σ 5 initial
The density is calculated using m/V. There is not a straightforward way to detect density in 1 step (as with geometry). The dubiety in the density would be given by:
Book by pycnometry
Pycnometry is a technique that uses the density relationship between book and mass, and the vessel used is called a pycnometer . To perform pycnometry measurements, the mass of the cylinder and the mass of a flask filled with water to a mark (A, Fig. 3) are recorded. The cylinder is then inserted into the flask. Water is displaced when the cylinder is inserted. The volume of water displaced is removed past pipet, thereby restoring the water level to the marker (B). The combined mass of the flask, remaining water, and cylinder is then measured.
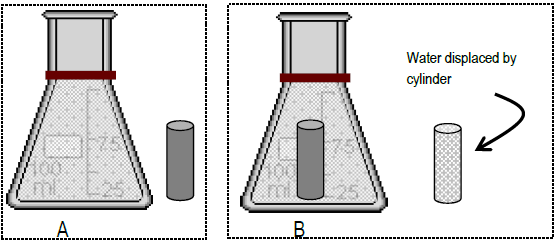
Figure 3
The sums of the masses before and later are equal. The massA, the massB, and the masscylinder were all measured on the balance. There is only one unknown in the equation - the mass of the displaced water.
( 4a )
mass A + mass cylinder = mass B + mass displaced water mass displaced water = mass A + mass cylinder − mass B
The volume of h2o removed is equal to the volume of the cylinder. Massh2o tin can exist converted to volume using the density of water.
( 4b )
5 displacedwater = Five cylinder = mass displaced water / density water
The density of the cylinder is calculated using mcyl/Vcyl. The uncertainty calculation requires a few steps and assumptions. The volume of the cylinder was equal to the volume of the water. 5h2o was based on the three mass measurements - the mass of the cylinder, of A, and of B. The dubiety in masscylinder comes from the residue reading. The uncertainty associated with massA and massB depends on your ability to precisely adjust the level of the water to the mark at the exactly same place every time (calibration). By repeatedly filling the flask to the mark and taking the mass readings, the average mass of A and the standard deviation (the fluctuation in the mass due to variations in the exact liquid level) can exist establish.
( 4c )
k A , trial i + m A , trial 2 + chiliad A , trial iii +
# trials
( 4d )
σ thouA = ±
Presume the doubtfulness in the mass of both A and B is the same: mA ± σ mA; mB ± σ mA. The uncertainty in the mass of water displaced is adamant past mistake propagation:
( 4e )
σ k water = σ grand A + σ chiliad B + σ m cyl = σ thousand A + σ m A + σ m cyl
The density of water at room temperature is known quite precisely and is assumed to contribute negligible error (come across table at the cease of the lab), so dividing σ m, h2o past the density of h2o to requite σ V, water is acceptable. Since σ 5, h2o = σ V, cyl, the uncertainty in the density can be adamant.
You lot will use pycnometry in parts 4 and 5 to determine the volume and/or density of a hollow cylinder and of a mixed cylinder.
Book of a void inside a hollow cylinder
A hollow cylinder has an empty infinite inside.
Figure 4
The volume of the cylinder is comprised of the volume of metal and the book of the void inside.
( 5a )
Vcyl = Vmetal + 5void → Fivevoid = Vcyl - Vmetal
Vcyl is determined by pyncometry. The volume occupied by the metal can be adamant using the mass of the cylinder (which is due to only the metallic, not the void) and the density of the metal, which was adamant previously in the lab (either Al or contumely, depending on the cylinder). Utilize the value for density that is closest to the literature values - 2.70 m/cmiii for Al; betwixt 8 and ix grand/cm3 for brass.
( 5b )
V metal = No error propagation is required
Percent limerick of a mixed cylinder
The full mass of the cylinder, one thousandcyl, is the sum of the mass of Al and brass (grandAl + mbrass). In terms of fractional composition, this would be Xmcyl and (1 - X)mcyl, respectively, where X is the Al fraction and (1-Ten) is the brass fraction (the remainder). The cylinder volume is determined by pycnometry and is the sum of the volumes of the two metals:
( 6a )
V cyl = V Al + V contumely
Supplant each volume past its mass divided past its density using V=1000/ ρ :
Replace the masses past the equivalent expressions in terms of 10 and mcyl:
Divide through by mcyl and replace Fivecyl/mcyl with 1/ ρ cyl:
Collect terms on the right-hand side that comprise X:
( 6e )
= Ten
Solve for Ten, the mass fraction of aluminum in the mixed cylinder.
This is the equation to utilize. Density of the cylinder is found by pycnometry. The densities of Al and brass have already been determined. When finding X:
a
Calculate each fraction in the equation, and so the differences, and and so the concluding ratio.
b
Use the densities of brass and aluminum adamant experimentally.
c
Discover X, and use X to decide the mass fraction of brass in the mixed cylinder, ane - X. 10 has a range of possible values from nix to one (0 - 100%). If your mixed cylinder's density is between that of aluminum and of brass, y'all should calculate a percent of aluminum that makes sense. For example, if the mixed cylinder has a density near that of Al, X should be nearly ane.
Equipment List
- cylinders: brass, aluminum, mixed brass/aluminum, and hollow
- Vernier caliper
- 50 mL Erlenmeyer flask, 100 mL graduated cylinder, 400 mL beaker
- lab marker
- Pasteur pipet
- thermometer
Procedure
Note: IF YOU WORK WITH Another Fix OF PARTNERS, Brand Certain Yous Record ALL DATA. YOU WILL Not BE ABLE TO Complete THE DATA Analysis IF YOUR Information TABLES ARE INCOMPLETE. As well Check THAT Information MAKES SENSE. Parts ane-3. Density of aluminum and brass cylinders using three different methods of volume measurement
Office 0: Measure out metal cylinder masses.
1
Obtain four cylinders - brass, aluminum (solid cylinders marked Due south), hollow (marked H), mixed contumely/aluminum (marked P for "plugged"). Return cylinders to the stockroom at the end of lab.
2
Record the cylinders' numbers.
3
Record the masses of the cylinders on the analytical balance to the 0.0001 thou (the uncertainities in your cylinders' masses are ±0.0001 yard). You volition use these masses throughout the experiment.
Part 1: Volume past Geometry
ane
Measure the diameter and length of each cylinder using the Vernier calipers. Your TA will assist you lot if you need it. Record the values to the 0.01 cm (each measurement is ±0.01 cm).
2
Determine the density of the cylinder. Observe the uncertainity using mistake propagation.
Role ii: Volume by Displacement
1
Put enough water to cover the metal cylinder into a 100-mL graduated cylinder and record the volume. The graduated cylinder is non very precise; readings will exist ±0.five mL (the digit in the tenths place will either be a five or a 0).
ii
Carefully slide the metallic cylinder down the side of the graduated cylinder into the water. Tossing it in can break the bottom of the graduated cylinder.
3
With the metallic cylinder completely submerged, record the new volume reading (to ±0.v mL).
4
Determine the volume of the metal cylinder. Calculate the dubiousness in your volume using error propagation.
5
Determine the density of the cylinder. Calculate the uncertainty using error propagation.
Role 3: Volume by Pycnometry
1
Fill up a 400 mL chalice with h2o and measure its temperature. Use this water throughout the experiment. Assume that the density of h2o makes a negligible contribution to the overall doubt in the values calculated.
two
Make your pycnometer.
-
a
Describe a ring midway upwardly the neck of a 50 mL Erlenmeyer flask with a waterproof marking or wax crayon, as shown below. -
b
Invert the flask on the tabular array; hold marker on top of something solid; and, rotate the flask while marking the cervix at a constant height.
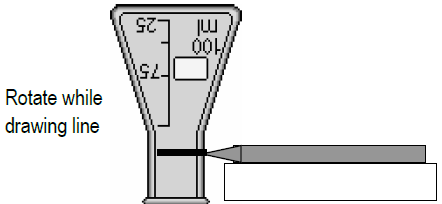
Figure 5
3
Calibrate your pycnometer. How well can you adjust the h2o'south meniscus to the top of the line fatigued? Precise filling to that marking increases reproducibility (and data quality). Exercise with the pycnometer before making measurements. Your TA volition demonstrate. The pycnometer filled with water to the mark is called 'A'.
-
a
Utilise a disposable pipet to add and remove drops of water to adjust the meniscus to the top of the line. -
b
Tape the mass of the flask and water. No drops should appear on the neck of the flask above the water line. -
c
Cascade out the water into your 400-mL beaker; refill to the marker; reweigh. -
d
Repeat step c until you lot accept 3 similar values for the mass of 'A'. -
e
Determine the average mA and its standard deviation ( σ mA). The standard divergence, σ mA, reflects your ability to reproducibly fill the pycnometer to the same place every fourth dimension you use it; σ mA is the uncertainity of the pycnometer and should be read with the average mass of 'A' equally well as the mass of 'B' (parts iii, 4, and 5). -
f
If you share data with some other set of students, make sure to tape their calibration data as well. You must apply the correct calibration outcome with the advisable data. In your lab notebook, label which cylinders become with which pycnometer calibration.
4
Indirectly measure the mass of water displaced past your solid cylinders. The pycnometer containing the metallic cylinder with water filled to the mark is called 'B'.
-
a
Carefully insert a metallic cylinder, fill up with water to the mark, and record the mass (the flask with water and cylinder). -
b
Repeat filling and weighing several times until the information appears reproducible. -
c
Calculate the mass of the water removed. Catechumen this mass to volume by dividing past the density of h2o (employ a precise value, specific to the water's temperature). This volume equals the volume of the metal cylinder. - Calculate the uncertainty in the mass of water removed using error propagation. Catechumen this mass to volume units by dividing by the density of water (utilize a precise value, specific to the water'south temperature). This value equals the uncertainity in the book of the metallic cylinder.
-
e
Echo with the other cylinders every bit instructed.
5
Determine the density of each cylinder. Include the uncertainties.
Delight exercise not throw the metal cylinders away. Please return them to the reagent demote. Please put disposable pipets (and any broken glassware) in cleaved glass containers, not the trash can. You volition lose points for inappropriate disposal.
Part iv: Decide Void Volume in a Hollow Cylinder by Pycnometry
i
For the hollow cylinder, tape identity of metallic of the hollow cylinder (either aluminum or brass). Yous recorded its mass at the commencement of the experiment.
two
Insert the cylinder into the pycnometer; remove the water above the line, and tape the new mass.
3
Decide the volume of the cylinder and summate the volume of the void. No error propagation.
Part 5: Mass Fraction of Al and Brass Decision for Mixed-metallic Cylinder by Pycnometry
1
You recorded its mass of the mixed metal cylinder at the beginning of the experiment.
2
Insert the mixed Al/contumely cylinder into the pycnometer, remove the h2o above the line, and record the mass.
iii
Determine the density of the cylinder and the mass fractions of Al and of brass (X and one-X, respectively). No error propagation.
Reporting Results
Complete your lab summary or write a written report (as instructed).
Results / Sample Calculations
- Masses and volumes for solid cylinders by each method
- %fault relative to literature values ( ρ Al = two.70 g/cmthree; ρ brass = 8.44 thousand/cm3, depending on the alloy's composition)
- Void volume
- Mass fraction
- Mistake analysis for parts 1-3
Discussion
- What you found out (refer to results tables) and how for all 5 parts
- What were the major experimental sources of error?
- Compare the iii methods used to make up one's mind volume - which method was more than authentic and why? Which was most precise?
- What could be washed to improve the precision in whatsoever or all of the methods?
- How does the instrument error compare to standard deviation error?
Review Questions
Whole degrees are listed down the left hand side of the tabular array, while tenths of a degree are listed across the height. And then to find the density of water at 5.4 °C, observe the whole degree past searching down the left hand column to 'v'. So slide across that row to '0.four'. The density of water at v.4 °C is 0.999957 yard/mL.
An Unknown Rectangular Substance Measures,
Source: https://www.webassign.net/labsgraceperiod/ucscgencheml1/lab_1/manual.html
Posted by: kellylithen.blogspot.com


0 Response to "An Unknown Rectangular Substance Measures"
Post a Comment